Galvanising transformer radiators will improve their life expectancy
By EPR Magazine Editorial February 10, 2023 3:35 pm IST
By EPR Magazine Editorial February 10, 2023 3:35 pm IST
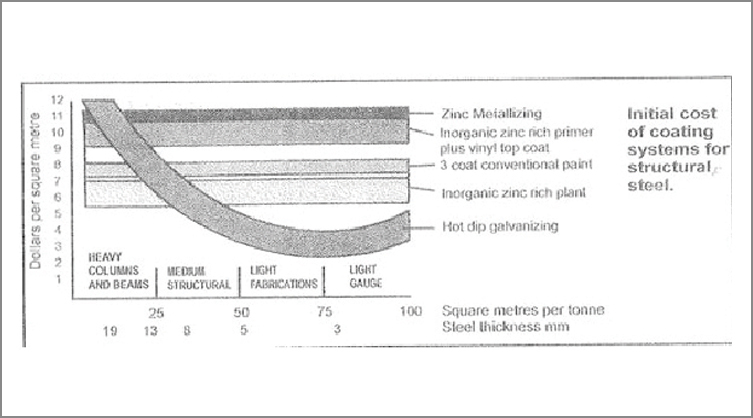
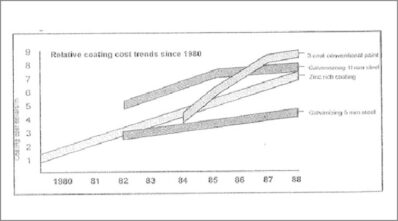
Power transformers are manufactured with a combination of HR and CR steel, which are long-lasting constructions with an expected life span of up to 25–40 years. Radiators with several elements made from 1 mm to 1.2 mm CR steel must withstand the corrosive attack due to environmental conditions. Compared to conventional corrosion protection, hot-dip galvanising is a long-lasting and durable type of corrosion protection. As shown in Figure 1, the corrosion protection of a hot-dip galvanised radiator could have an estimated life of up to 40 years. However, conventional coatings must be renewed every 4–5 years. If galvanising is used for corrosion protection, it may last up to 25–40 years, equal to the lifespan of a power transformer.
How zinc protects the radiator
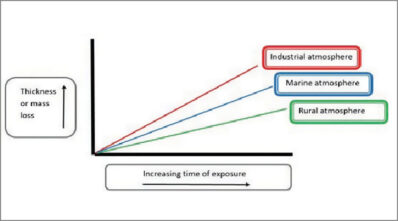
Steel is a very versatile product, and radiators are produced with steel. Unfortunately, it is prone to rusting, a phenomenon that causes the surface to become unsightly and, over time, may contribute to product failure. For this reason, radiators are protected by various methods ranging from internal alloying, e.g., stainless steel, to coating with galvanised coatings and/or paints. Corrosion is an electrochemical process that, in the case of steel, oxidises the iron in the steel and causes the steel to become thinner over time. Oxidation, or rusting, occurs due to the chemical reaction between steel and oxygen. Oxygen is always present in the air or can be dissolved in moisture on the surface of the steel sheet. During the rusting process, steel is consumed during the corrosion reaction, converting iron into corrosion products. In the case of most low-carbon steel sheet products, iron oxide (rust) develops on the surface and is not protective because it does not form a continuous, adherent film. Instead, it spalls, exposing fresh iron to the atmosphere, which, in turn, allows more corrosion to occur. This aspect of steel behaviour is undesirable, both aesthetically and in terms of service life. Eventually, often sooner than desired, steel corrodes sufficiently to unduly shorten its service life, i.e., loss of structural strength or perforation and water intrusion.
We use cookies to personalize your experience. By continuing to visit this website you agree to our Terms & Conditions, Privacy Policy and Cookie Policy.